| Drug Name |
V-102862
|
| Synonyms |
CO 102862; UNII-0KN11H90GF; 181144-66-1; V-102862; CHEMBL287833; 0KN11H90GF; 4-(4-fluorophenoxy)benzaldehyde semicarbazone; (E)-2-(4-(4-fluorophenoxy)benz-ylidene)hydrazinecarboxamide; V102862; 2-(4-(4-Fluorophenoxy)benzylidene)hydrazinecarboxamide; 2-((4-(4-Fluorophenoxy)phenyl)methylene)hydrazinecarboxamide; CO-102862; 2-[[4-(4-Fluorophenoxy)phenyl]methylene]hydrazinecarboxamide; Hydrazinecarboxamide, 2-((4-(4-fluorophenoxy)phenyl)methylene)-; CHEBI:92156; MolPort-022-863-701; BDBM50141073; AKOS016004881; NCGC00167807-01
|
| Drug Type |
Small molecular drug
|
| Structure |
|
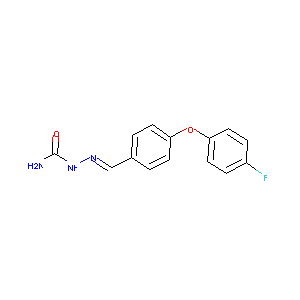 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
273.26 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.1 |
| Rotatable Bond Count (rotbonds) |
4 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| Chemical Identifiers |
- Formula
- C14H12FN3O2
- IUPAC Name
[(E)-[4-(4-fluorophenoxy)phenyl]methylideneamino]urea - Canonical SMILES
-
C1=CC(=CC=C1/C=N/NC(=O)N)OC2=CC=C(C=C2)F
- InChI
-
InChI=1S/C14H12FN3O2/c15-11-3-7-13(8-4-11)20-12-5-1-10(2-6-12)9-17-18-14(16)19/h1-9H,(H3,16,18,19)/b17-9+
- InChIKey
-
MHUUDVZSPFRUSK-RQZCQDPDSA-N
|
| Cross-matching ID |
- PubChem CID
- 9816959
- ChEBI ID
-
- CAS Number
-
- TTD ID
- D07EQF
|
|
|
|
|
|
|
|


